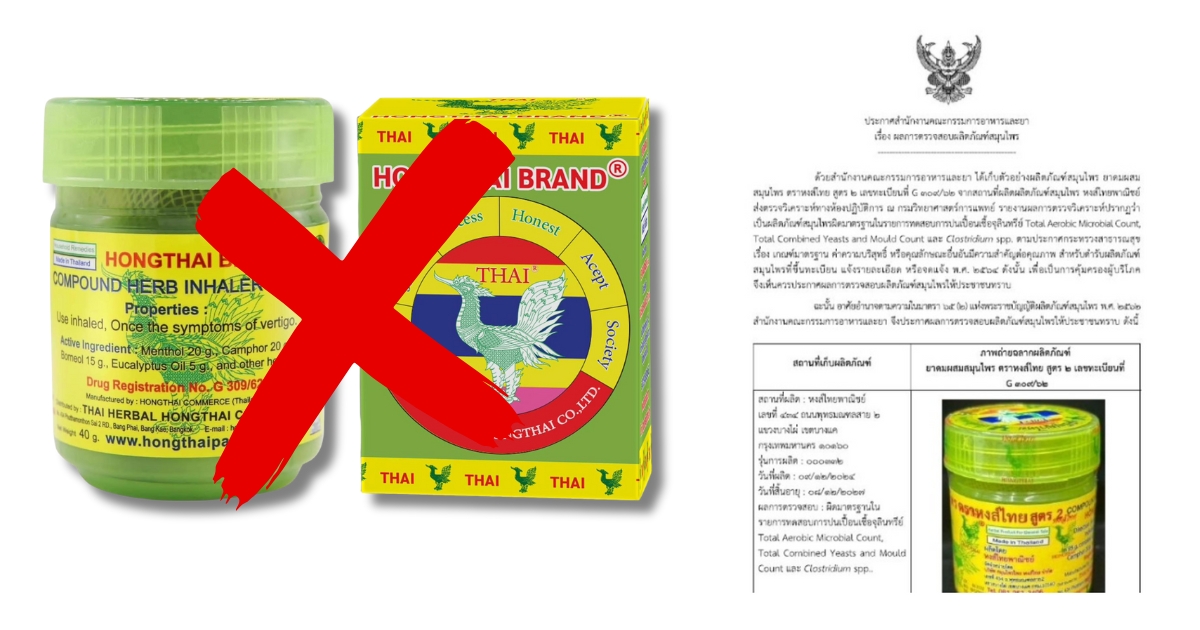
The Thai Food and Drug Administration (FDA) has issued an urgent public health warning and mandated the recall of a specific batch of the highly popular Hong Thai Herbal Inhaler Formula 2 (Reg. No. G 309/62) after it failed critical safety tests due to severe microbial contamination.
The FDA found that the product batch in question contained unacceptable levels of bacteria, yeast, and mold. Most critically, testing detected Clostridium spp., a genus of bacteria known for producing robust spores and highly potent toxins, raising serious public health concerns regarding the safety of an inhaled product.
The Contamination Crisis
The Hong Thai herbal inhaler is a staple in Thailand and across Southeast Asia, widely used for nasal congestion, dizziness, and headache relief. Its popularity and method of use—direct inhalation—make the discovery of contamination particularly alarming.
The contaminants detected weren’t just signs of poor hygiene; they posed a direct threat:
- High Microbial Loads: Excessive general bacteria, yeast, and mold counts indicate a significant breakdown in manufacturing cleanliness, which can lead to allergic reactions and respiratory irritation, especially for individuals with asthma or other underlying conditions.
- The Clostridium Threat: The presence of Clostridium spp. is a major regulatory violation. This family of bacteria includes species that can cause severe, sometimes fatal, diseases like botulism or gas gangrene. Introducing these spores directly into the respiratory system through an inhaler creates an unacceptable and potentially fatal risk profile.
Consumer Action Required
The FDA warning applies only to the specific batch identified below. Consumers are strongly advised to stop using this product immediately and return it to the place of purchase for a refund or replacement.
Here are the details of the affected product:
- Product: Hong Thai Herbal Inhaler Formula 2
- Registration No.: G 309/62
- Affected Batch No.:000332
- Manufactured Date: December 9, 2024
- Expiry Date: December 8, 2027
The manufacturer, Thai Herbal Hong Thai, confirmed that it has acknowledged the FDA’s findings and initiated a full recall and destruction of the affected units. The company has also announced plans to significantly upgrade its quality control procedures, including adding UV sterilization to its process, to ensure future product safety.
The Wider Lesson for Traditional Medicine
This incident highlights the critical need for traditional herbal products, particularly those administered via sensitive routes like inhalation, to adhere to the same stringent pharmaceutical-grade safety standards (Good Manufacturing Practices, or GMP) as modern medicine.
Botanical raw materials naturally carry a high bioburden, including soil-based organisms like Clostridium spores. For companies to guarantee consumer safety, they must employ rigorous testing and validated sterilization methods such as heat or specialized irradiation, capable of inactivating these robust, spore-forming pathogens before the product ever reaches the consumer.
The FDA’s swift enforcement action signals a clear message: purity and quality are non-negotiable standards for all products in the Thai healthcare market.